

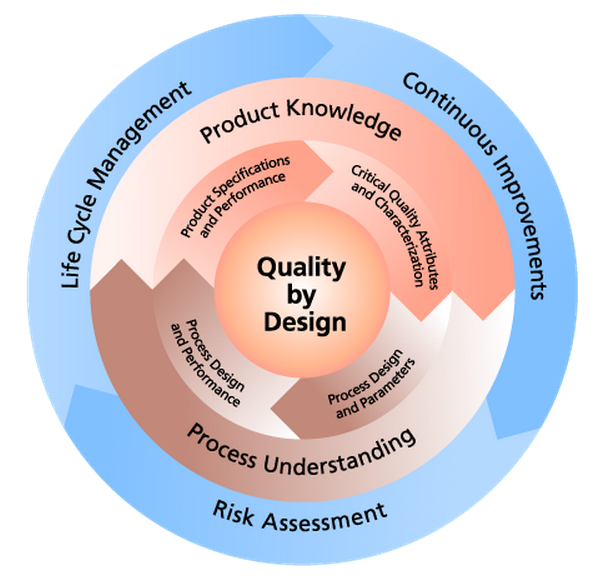
Unlike the empirical-based methods used in traditional product development and manufacturing methods, QbD is a scientific, risk-based approach that focuses on designing quality into a product from the earliest stages of planning to prevent quality failures from ever occurring and more readily address them if they do occur. QbD is now on the rise among forward-thinking companies of all sizes, particularly as the required knowledge, technology and tools become more established and widely available. But what we do know is that the FDA, EMA and other key regulatory authorities support a risk-based approach and the inclusion of QbD principles in the development and production of drug products. QbD is also thoroughly addressed in the latest ICH Quality Guidance documents Q8 to Q11, each covering different aspects of the concept.
The pharmaceutical Quality by Design (QbD) is a systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management. Quality by Design (QbD) is emerging to enhance the assurance of safe, effective drug supply to the consumer, and also offers promise to significantly improve manufacturing quality performance. While QbD will provide better design predictions, there is also a strong recognition that industrial scale-up and commercial manufacturing experience provides new and very important knowledge about the process and the raw materials used therein.